Important Safety Information
Caution: Federal law restricts this device to sale by or on the order of a physician.
Indication: The Pulmonx Zephyr® Endobronchial Valves are implantable bronchial valves indicated for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation.
Contraindication: The Zephyr Valve is contraindicated for: Patients for whom bronchoscopic procedures are contraindicated; Patients with evidence of active pulmonary infection; Patients with known allergies to Nitinol (nickel-titanium) or its constituent metals (nickel or titanium); Patients with known allergies to silicone; Patients who have not quit smoking; Patients with large bullae encompassing greater than 30% of either lung.
Warnings: The Zephyr Valve should be used with caution and only after careful consideration in patients with: Prior lung transplant, LVRS, median sternotomy, or lobectomy; Congestive heart failure or recent myocardial infarction; FEV1 <15% of predicted value. The Zephyr EDC handle contains strong permanent magnets. To avoid interference and possible patient/clinician harm, keep it more than 2 inches away from medical devices that could be affected by the magnetic fields, including, but not limited to pacemakers, cochlear implants, and neurostimulators. Additionally, strong magnetic fields may cause damage to magnetic data storage media and electronic equipment if brought within two inches of the Zephyr EDC..
General Precautions: Read all labels and instructions prior to use. Use is restricted to a physician trained in the use of this device. The Zephyr Valve and EDC are intended for single-patient use only. Do not re-sterilize. No assurance of sterility can be made if devices are re-used. Do not attempt to reload a Zephyr Valve. Do not use the device if the sterilization barrier has been damaged or if the device is dropped after removal from sterile packaging. Performance of the Zephyr Valve has not been assessed in physiological conditions unique to air leak patients with an open thoracic window (also known as a Claggett Window or Eloesser Flap). The Zephyr Valve device may be subject to fracture due to unusual physical forces in this setting. Safety in uncontrolled pulmonary hypertension has not been evaluated. The Zephyr Valve was evaluated in patients with heterogeneous emphysema in the pivotal trial, LIBERATE Study. Limited data on the use of this device in homogeneous emphysema patients come from the OUS IMPACT trial.
Adverse Effects: Probable adverse events include, but are not limited to, the following: Acute respiratory distress syndrome; Airway erosion; Airway stenosis; Aphonia; Bowel function impairment; Bronchitis; Bronchospasm; Chest Pain; COPD exacerbation; Cough; Death; Disorientation/anxiety; Dyspnea; Empyema; Epistaxis; Fever; Granulation tissue / ulceration formation; Headache; Heart arrhythmia; Heart Failure; Hematoma; Hemoptysis; Hemothorax; Hypotension; Hypercapnia; Hypoxemia; Iatrogenic injuries; Impaired lung function; Increased mucus secretions; Infection; Insomnia; Musculoskeletal event; Myocardial infarction; Nausea/vomiting; Pain; Pleural effusion; Pneumonia; Pneumothorax; Pulmonary embolism; Pulmonary shunting; Residual volume increase; Respiratory failure; Sepsis; Shortness of breath; Sore throat; Stroke/CVA/TIA; Systemic inflammatory response syndrome (SIRS); Valve migration/expectoration; Vocal cord injury; Wheeze or whistling.
Prior to use, please reference the Zephyr EBV System Instructions for more information on indications, contraindications, warnings, all precautions, and adverse events.
US-EN-1023-v1


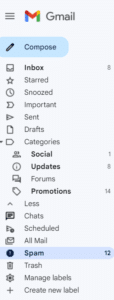
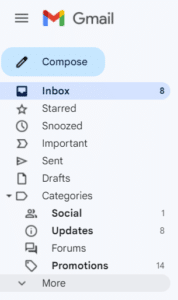 If you do not see an email from “Val at Zephyr Valve” in your inbox within 15 minutes of signing up, go to your “Spam” folder within Gmail. (You may have to click "More" to see the Spam folder.) Select the email from "Val at Zephyr Valve."
There will be a gray box at the top that says “Why is this message in spam?” Click the “Report Not Spam” button. This will move the email to your inbox.
If you do not see an email from “Val at Zephyr Valve” in your inbox within 15 minutes of signing up, go to your “Spam” folder within Gmail. (You may have to click "More" to see the Spam folder.) Select the email from "Val at Zephyr Valve."
There will be a gray box at the top that says “Why is this message in spam?” Click the “Report Not Spam” button. This will move the email to your inbox.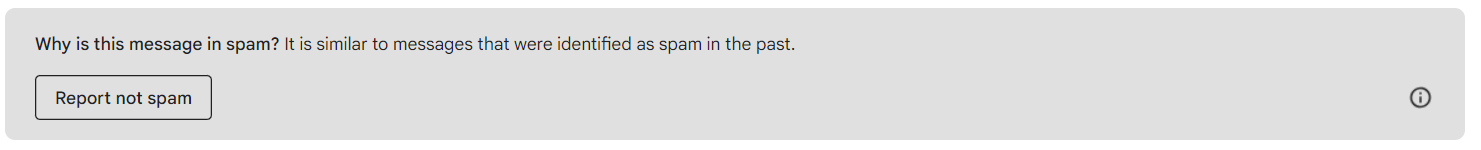 Go back to your inbox and open the email. Click on the icon next to “Val at Zephyr Valve”, you’ll see a person icon with a “+”. Click that, and you can add Val to your Contacts list. If you don’t receive a second email from us in a few days, please check your Spam folder again.
Go back to your inbox and open the email. Click on the icon next to “Val at Zephyr Valve”, you’ll see a person icon with a “+”. Click that, and you can add Val to your Contacts list. If you don’t receive a second email from us in a few days, please check your Spam folder again.
